Quick Hit Summary
Everyone knows that training is a key component of the performance equation. However, individuals often forget the most important part of the equation, sufficient recovery. This is achieved through adequate rest + proper nutrition. Due to the highly catabolic nature of physical exercise, influenced by both mechanical stress and hormones, it’s important to quickly switch your body from a catabolic to anabolic state as soon as possible post workout. In doing so, you’ll speed up the recovery process, priming your body for great gains during the ensuing workout. How to best achieve this conversion will be discussed in upcoming articles.
The Performance Equation: Hard Work + Proper Recovery = Success

Is your post workout nutrition measuring up?
When seeking to improve physical performance, there are two obvious variables that one can manipulate; the workout itself and the remaining 20+ hours of the day. If I had to determine the overall proportion that each piece factors into the performance equation, I’d go with a 70:30 split, with the non-workout hours having the greatest impact on performance. There are a few specific time periods when nutrition can have a significant impact on post workout recovery. In this article, I’d like to address one of them, the first 2-4 hours following a workout. During this time period, two important goals must be met. First, one must assist the body as it tries to go from a catabolic state (breaking down human tissue) to an anabolic state (building up human tissue). The second goal, which is really just an extension of the first goal, is to replenish glycogen (carbohydrate) stores that have been depleted during exercise.
Factors Contributing to Muscle Catabolism
Following an intense exercise session, ones muscles are in a state of catabolic, self destruction. Two catabolic fronts are set up which directly oppose muscle growth. The first front is the mechanical stress of the actual exercise, which is directly responsible for the micro-tears seen in skeletal muscle following a training session15. The second front results from an increased release of catabolic hormones such as cortisol, which acts to break down muscle tissue (particularly type II fibers) while simultaneously decreasing the rate of protein synthesis1. This elevation in cortisol during and immediately post exercise occurs in resistance, endurance and intermittent/interval training sessions123.
Although cortisol and micro-tears may seem detrimental to muscle growth, they’re both required to some degree to elicit a training response. Micro-tears cause the mild inflammation that is required to stimulate the physiological processes required for muscle growth and adaptation4. Although it serves other functions, one of the main functions of cortisol is to maintain blood glucose levels when they start to drop. It accomplishes this by breaking skeletal muscle down into amino acids, which are then converted by the liver into glucose before being released back into the bloodstream. Thus, athletes, specifically endurance based, can train/compete at a higher intensity.
Cortisol and Exercise
Training Intensity
One of the key factors influencing the rise in cortisol levels both during and after an exercise session is training intensity. In resistance training, Smilios et al. demonstrated that the rep/set scheme affected post exercise cortisol levels5. In their study, 11 trained men (mean age-23) completed 8 resistance training sessions with the following focuses:
- Strength (5 rep sets, starting at 80-88% 1RM, 3 min rest intervals); see **
- Hypertrophy (10 rep sets, starting at 68-75% 1RM, 2 min rest intervals); see **
- Strength & Endurance (15 rep sets, starting at 52-60% 1RM, 1 minute rest) see **
- **Additionally, they also completed 2, 4 and 6 sets of each exercise protocol spaced 1 week apart from each other (except strength endurance that was only completed at 2 and 4 sets).
Results of the study demonstrated that both hypertrophy and strength endurance sessions increased cortisol levels compared to baseline measures. No significant differences were seen in the max strength session. In addition, increasing from 2 to 4 sets, elevated the cortisol response even further in both the strength endurance and hypertrophy sessions. However, no additional increase was seen in cortisol levels when increasing from 4 to 6 sets. It should be noted that this study took place over an 8 week period and some of the responses seen may have been due to a training effect rather than the exercise protocol. However, the various exercise sessions were completed in a random order in attempt to account for this confounding variable.
Training Mode
In a study involving 7 sedentary men (mean age- 28), 7 resistance trained men (mean age-23), and 8 endurance trained men (mean age- 31), Tremblay et al. investigated how training status affected the cortisol response in men following a jogging and resistance based circuit training sessions6. Participants first completed a 40 minute jogging session (50-55% VO2), followed a week later by a circuit training session (7 exercises, 10RM intensity). No predetermined number of circuits was set for the resistance training. Rather, each participant completed circuits until the caloric (kcal) expenditure equaled the number burned during the jogging session. The mean duration of the circuit training varied between 62-66 minutes.
Results of the study indicated that cortisol levels were significantly elevated 30 and 60 minutes following the start of the resistance training protocol vs. the control session (quiet rest)6. This held true regardless of one’s training background. When combining the results from all participants, over the entire 2 ½ hour monitoring period, a significantly higher cortisol level was present for all groups during and following the resistance training compared to the jogging or rest conditions. Researchers failed to find significant differences between the jogging and rest sessions. This was expected as it’s hypothesized that cortisol levels only rise during aerobic sessions with intensities >60-70% VO2max7 unless they’re of longer duration (see below).
Duration of Training Session
More recently, the exercise duration has been shown to be an independent factor affecting cortisol levels. In a 2nd study conducted by Tremblay et al., researchers had 8 endurance trained athletes run at 55%VO2max for 40, 80, and 120 minutes8. Although rises in cortisol were not present in the 40 or 80 minute session, significant elevations were observed during the 120 minute session. This occurred despite never surpassing the aforementioned 60-70%VO2max threshold7.
Although this might seem somewhat surprising, one must remember the role of cortisol. As previously mentioned, one of the functions of this hormone is to increase blood glucose levels (and break down fat for energy). During aerobic workouts of higher intensities (as measured by VO2) one cannot consume (ie-breathe in) enough oxygen to rely upon fat as the main source of energy9. Thus, at higher workout intensities, the body relies predominantly on carbohydrates as its main source of fuel. Muscle cells first tap into their own carbohydrate stores (muscle glycogen). Once muscle glycogen levels get low, cells increase glucose uptake from circulation. As glucose levels drop in the bloodstream, one’s adrenal glands are stimulated to release cortisol. At low exercise intensities, the body can consume enough oxygen to efficiently burn fat for energy. Although fat is the predominate source of energy at these intensities, a steady supply of glucose must be available to completely metabolize fat. Therefore, I hypothesize that enough carbohydrate was available during the 40 and 80 minute jogging sessions to allow this process to occur without depleting the blood glucose levels. On the other hand, during the 120 minute session, blood glucose levels may have started to dip a little bit, stimulating cortisol release.
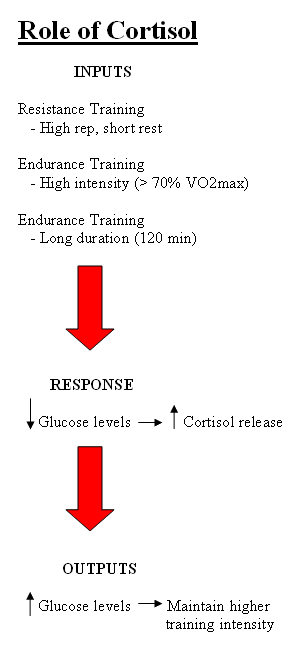
Figure 1 Influences and effects of cortisol with respect to glucose. Although not diagrammed, please note that cortisol also breaks down fat, increasing its availability for energy use as well.
Glycogen Stores & Performance
Aerobic Performance
As alluded to above, glucose, which is stored in both the liver and muscle tissue as glycogen, is critical for aerobic performance. The human body stores ~350 kcal (87.5g) of glycogen in the liver and an additional ~ 1,400 kcal (350g) of glycogen in muscle9. Although this may seem like a lot of glycogen, one must remember that skeletal muscle is not the only tissue that requires glycogen for energy. For instance, the human brain relies almost entirely on glucose for energy at rest and light to moderate activity1011. Although it will use lactate (produced by working muscles) as a supplemental energy source at higher workout intensities, glucose still serves as a primary source of kcal. As glycogen levels drop during endurance events, athletes are forced to rely heavily on fat. Without a large supply of oxygen present, it is impossible to burn fat for energy. To allow enough oxygen to reach the muscle, one must slow down the speed at which they are moving. The extreme side of glycogen depletion is often scene in endurance events such as marathons and is referred to as “bonking” or “hitting the wall.” This occurs when glycogen stores are depleted, and one must rely predominantly on fat for energy. Under these circumstances, one can go from a smooth jog to a slow, painful walk.
Anaerobic Performance
During intensive resistance training sessions, the majority of energy derived is from muscle glycogen sources. In fact, Macdougal et al. demonstrated that in 1 set of bicep curls to failure, lasting a total of 37 seconds, glycolysis (breakdown of glucose for energy) accounted for 82% of the total energy used12. (The other energy system that contributes that generally contributes to resistance training is the ATP-PC system, which provides energy for ~10 seconds before it’s exhausted9. The discussion of the ATP-PC energy system is beyond the scope of this article). However, it should be noted that if one’s muscle glycogen stores are full at the start of a resistance training session, one should not have to worry decreases in performance due to a lack of glycogen. Even exhaustive resistance training does not completely deplete muscle glycogen stores. In the previously mentioned study by Macdougal et al., the research team also found that the exhaustive set of arm curls decreased muscle glycogen content in the biceps by 12%12. In another study, Robergs et al. examined how 6 sets of exhaustive leg extensions affected muscle glycogen content in the rectus femoris (quad muscle) of 8 resistance trained individuals (mean age- 23)13. Each participant completed 6 sets of exhaustive leg extensions with 2 minute rest intervals between sets. Each individual completed a mean of 12-13 reps/set. Results indicated that following the final set, muscle glycogen content decreased by 38% compared to baseline values.
Although muscle glycogen stores are not associated with decreased performance during a bout of resistance training, many sport athletes use resistance training as a “means to an end.” It’s not uncommon for competitive athletes to participate in a resistance training session during the AM hours, only to be followed 4-8 hours later by a their sport practice. Although they will not exhaust their glycogen supplies during the resistance session, failure to replenish this store may impair physical performance during the ensuing hockey, basketball, etc, practice.
Bottom Line
The main goals of post-workout nutrition are to create an anabolic environment within one’s body to support tissue growth. In the process of accomplishing the first goal, one will often achieve the second goal, replenishing glycogen stores.
How these principles apple to both endurance & strength/ball/speed sports will be discussed in future articles.
References
1 Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339-61.
2 Cunniffe B, Hore AJ, Whitcombe DM, Jones KP, Baker JS, Davies B.
Time course of changes in immuneoendocrine markers following an international rugby game. Eur J Appl Physiol. 2009 Sep 16. [Epub ahead of print].
3 Jaya T. Venkatraman1 and David R. Pendergast. Effect of Dietary Intake on Immune Function in Athletes. Sports Med 2002; 32 (5): 323-337.
4 Goldspink G, Harridge S. Cellular and Molecular Aspects of Adaptation in Skeletal Muscle. In: Komi PV, editor.Strength and power in sport. 2nd ed. Malden (MA): Blackwell Scientific Publications, 2003: 231-251.
5 Smilios I, Pilianidis T, Karamouzis M, Tokmakidis SP. Hormonal responses after various resistance exercise protocols. Med Sci Sports Exerc. 2003 Apr;35(4):644-54p.
6 Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol. 2004 Feb;96(2):531-9. Epub 2003 Sep 26.
7 Viru A, Smirnova T, Karelson K, Snegovskaya V, Viru M. Determinants and modulators of hormonal responses to exercise. Biol Sport 13:169–187. (1996).
8 Tremblay MS, Copeland JL, Van Helder W. Influence of exercise duration on post-exercise steroid hormone responses in trained males. Eur J Appl Physiol. 2005 Aug;94(5-6):505-13. Epub 2005 Jun 8.
9 Benardot, D. Advanced Sports Nutrition.Human Kinetics. Chicago, Ill. 2006.
10 Dalsgaard MK, Ogoh S, Dawson EA, Yoshiga CC, Quistorff B, Secher NH. Cerebral carbohydrate cost of physical exertion in humans. Am J Physiol Regul Integr Comp Physiol. 2004 Sep;287(3):R534-40. Epub 2004 May 20.
11 Secher NH, Seifert T, and Van Lieshout JJ.Cerebral blood flow and metabolism during exercise: implications for fatigue Neils H. Secher, Thomas Seifert, and Johannes J. Van Lieshout.
12 MacDougall JD, Ray S, Sale DG, McCartney N, Lee P, Garner S. Muscle substrate utilization and lactate production.Can J Appl Physiol. 1999 Jun;24(3):209-15.
13 Robergs RA, Pearson DR, Costill DL, Fink WJ, Pascoe DD, Benedict MA, Lambert CP, Zachweija JJ. Muscle glycogenolysis during differing intensities of weight-resistance exercise. J Appl Physiol. 1991 Apr;70(4):1700-6.
14 Lambert CP, Flynn MG.Fatigue during high-intensity intermittent exercise: application to bodybuilding. Sports Med. 2002;32(8):511-22.
15 Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002 Nov;81(11 Suppl):S52-69.
16 Photo by kteague. Accessed June 14, 2010 from: flickr.com/photos/49503205198@N01/3335735763
